Bioavailability basically means the amount of active ingredient that remains available after digestion. The result of every study has been a demonstrated increase in bioavailability upon using the liposomal form of supplementation.
This blog post will explain how these bioavailability studies are performed. We will also explain how we interpret the results.
Step 1: Deciding on the nutrient to be tested
We have studied the bioavailability of our Vitamin C, magnesium and vitamin D3 formulations thus far.
Step 2: Sourcing “standard” products on the market
After deciding upon which liposomal formulation we want to test, the next step is to choose an “equivalent” supplement existing on the market. The supplement
- must have the same concentration of the active ingredient as our formulation
- should be orally administered.
The supplement MUST BE a widely consumed form such as a liquid, capsule or tablet. This is then referred to as the “standard” or “control” supplement. This is usually a traditional, non-liposomal supplement.
Where possible, we also compare our liposomal formulation to other liposomal formulations.
Step 3: Conducting the study
The bioavailability studies are conducted in association with Surya Research Clinic (India).
All studies are randomised controlled trials. This means an experiment performed to compare a “new” supplement against an existing form. Our liposomal formulations are considered to be the “new” form of supplementation, and are being compared to the traditional or “control” form of supplementation.
The RCT is the most rigorous and robust method of determining whether a given supplement indeed has the claimed effects (1).
In our studies, metabolically healthy volunteers of both sexes are enrolled in the trial. Participants are excluded from the study when:
- Age is less than 20 years or more than 50 years
- Any diagnosis of chronic condition(s) such as hypertension, diabetes etc.
- BMI (body mass index) is outside of the normal category range
- Presence of acute illness
- Use of drugs or dietary supplements on a frequent and/or mandatory basis
Every study group is randomly assigned an equal number of participants. Each group must have a minimum of 10 participants. The participants are not made aware of whether they belong to the liposomal or non-liposomal group.
Since many nutrients are available through one’s regular diet, we expect some amount of the test nutrient to already exist in the blood of the participants. This is known as the baseline plasma level of the nutrient being tested. Blood samples are taken from each participant before supplement administration to determine these baseline levels.
Through this, we can determine if the study groups start in a level playing field. The average baseline plasma levels of all study groups should be the same in order for us to be able to detect any changes due to supplementation.
Soon after baseline blood withdrawal, the study participants are administered their respective supplement form orally. Blood is subsequently withdrawn at regular, pre-determined intervals. This blood is processed and the concentration of the particular nutrient(s) at every time point is determined by sophisticated biochemical or biophysical methods. All analyses are performed in the laboratories of Surya Research Clinic.
Step 4: Evaluation of the study results
The study centre provides our R&D team with the results of the blood analyses for every participant, at every time point tested. We then plot these concentrations in a graph and perform various calculations as follows:
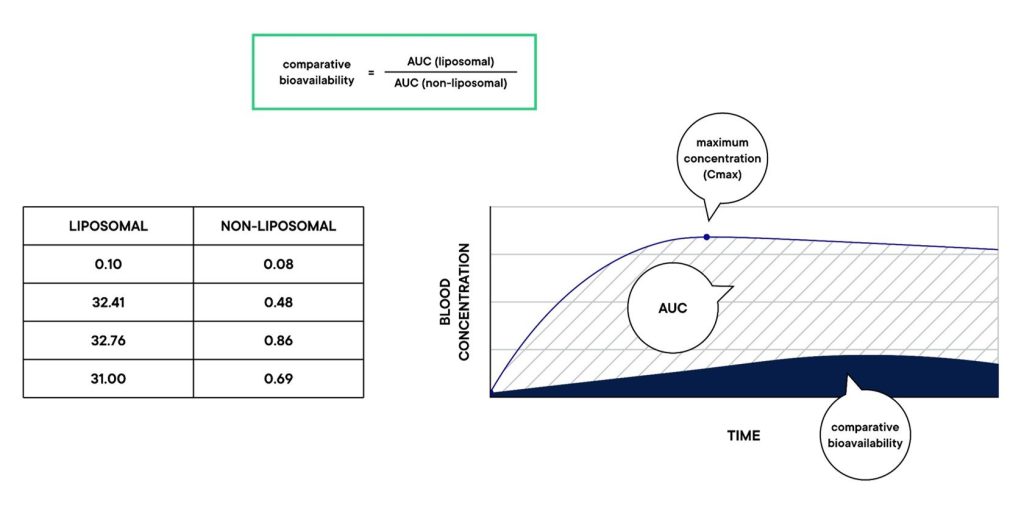
Cmax: This is the maximum ingredient concentration detected in the blood sample.
Tmax: This is the time at which the Cmax is reached.
AUC: This is the area under the plot of the plasma concentration of the nutrient versus the time after supplementation. It is an indication of the amount if the nutrient that reached the bloodstream.
Comparative bioavailability: A ratio of the AUCs between any two groups provides the comparative bioavailability between the two interventions. If the ratio is positive, it means that group A is more bioavailable than group B. The opposite is true if the ratio is negative.
The statistical significance of the above calculations is also plotted by our team. When a value is considered statistically significant, it means that the effect is “real” and not by chance.
Highly bioavailable of our supplements
From all the studies we have performed so far, the Mighty Kids liposomal formulations have always had the highest, statistically significant bioavailabilities.
Our liquid liposomal supplements are, therefore, a highly effective way of delivering nutrients into the bloodstream. This means that the nutritional and body-supportive potential of active ingredients can be unleashed with our liposomal formulations.
Key takeaways
- Bioavailability studies have been conducted on several of our liposomal formulations.
- The nutrients studied are based on consumer demand. Vitamin C, magnesium and vitamin D3 have been studied so far.
- Our liposomal formulations are compared to other liposomal and non-liposomal products of the same dosage. The average Cmax, Tmax, AUC and comparative oral bioavailabilities of all study groups are determined by a scientific team.
- The randomised controlled trial is the most rigorous and robust research method of determining whether a cause–effect relation exists between food supplementation and its outcome.
- Our liquid liposomal formulations have been determined as more bioavailable than all other comparative products.